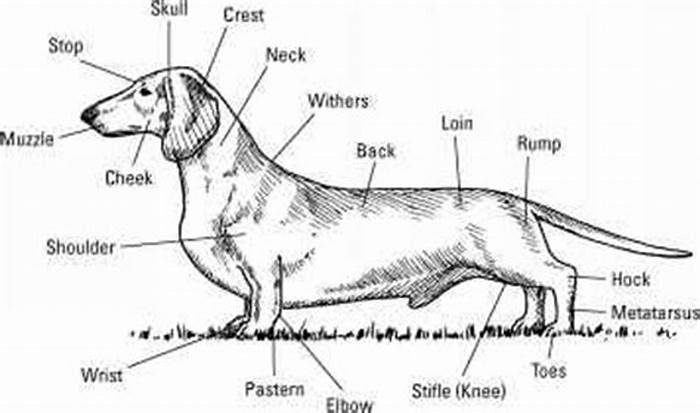
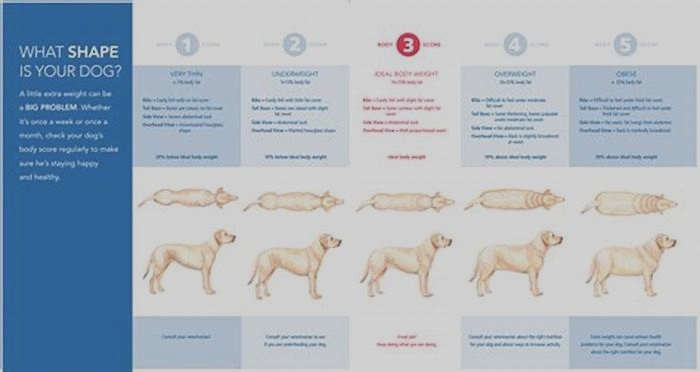
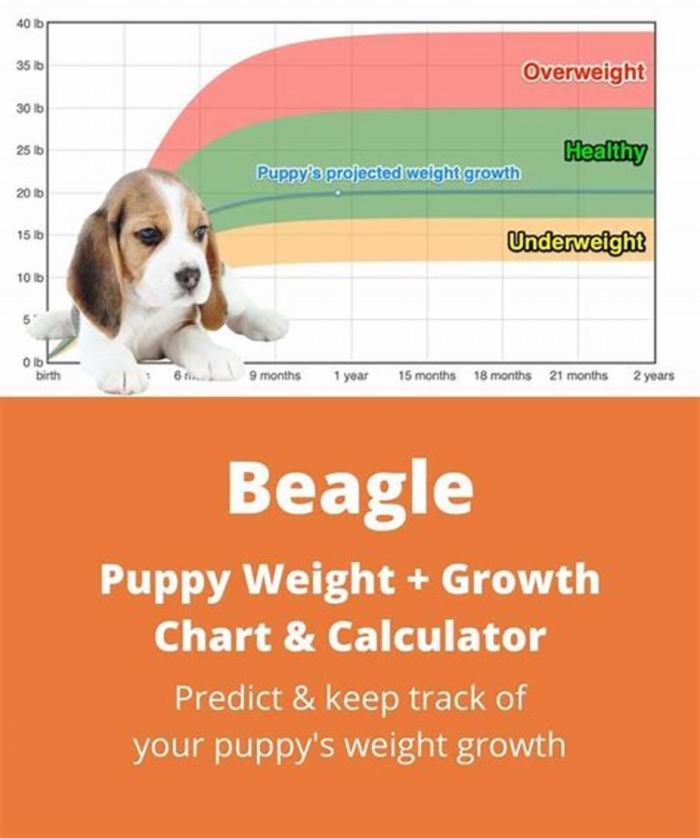
Understanding Beagle Body Composition Factors Influencing Weight
Changes in weight, body composition, and factors influencing energy balance among premenopausal breast cancer patients receiving adjuvant chemotherapy
Purpose: Weight gain is a common problem among breast cancer patients who receive adjuvant chemotherapy (CT). We undertook a study to determine the causes of this energy imbalance.
Patients and methods: Factors related to energy balance were assessed at baseline (within 3 weeks of diagnosis) and throughout 1 year postdiagnosis among 53 premenopausal women with operable breast carcinoma. Thirty-six patients received CT and 17 received only localized treatment (LT). Measures included body composition (dual energy x-ray absorptiometry), resting energy expenditure (REE; indirect calorimetry), dietary intake (2-day dietary recalls and food frequency questionnaires) and physical activity (physical activity records).
Results: Mean weight gain in the LT patients was 1.0 kg versus 2.1 kg in the CT group (P =.02). No significant differences between groups in trend over time were observed for REE and energy intake; however, a significant difference was noted for physical activity (P =.01). Several differences between groups in 1-year change scores were detected. The mean change (+/- SE) in LT versus CT groups and P values for uncontrolled/controlled (age, race, radiation therapy, baseline body mass index, and end point under consideration) analysis are as follows: percentage of body fat (-0.1 +/- 0.4 v +2.2 +/- 0.6%; P =.001/0.04); fat mass (+0.1 +/- 0.3 v +2.3 +/- 0.7 kg; P =.002/0.04); lean body mass (+0.8 +/- 0.2 v -0.4 +/- 0.3 kg; P =.02/0.30); and leg lean mass (+0.5 +/- 0.1 v -0.2 +/- 0.1 kg; P =.01/0.11).
Conclusion: These data do not support overeating as a cause of weight gain among breast cancer patients who receive CT. The data suggest, however, that CT-induced weight gain is distinctive and indicative of sarcopenic obesity (weight gain in the presence of lean tissue loss or absence of lean tissue gain). The development of sarcopenic obesity with evidence of reduced physical activity supports the need for interventions focused on exercise, especially resistance training in the lower body, to prevent weight gain.
Bookshelf
There are numerous factors that can influence body weight. The individual has no control over some of these factors, including developmental determinants, genetic makeup, gender, and age. Other factors that influence body weight over which the individual has potential control include level of physical activity, diet, and some environmental and social factors. This chapter explores the relationship between each of these factors and body weight.
DEVELOPMENTAL DETERMINANTS
It has been postulated that there are times during people's lives when exposure to certain factors may increase their risk for the onset of obesity. These times have been termed critical periods. If these critical periods, along with the influential factors, can be clearly defined, it may be possible to identify individuals at increased risk for the development and persistence of overweight and obesity in adulthood. The prenatal period, the period of adiposity rebound, and adolescence have been proposed as critical periods in childhood (Dietz, 1994); pregnancy and the immediate postpartum period have been proposed as critical periods for women in adulthood.
Prenatal Factors
Although the data are subject to a variety of interpretations, it has been documented in both animals and humans that females who are severely food restricted during the first one to two trimesters of pregnancy have progeny who have a higher prevalence of obesity, diabetes, insulin resistance, and hypertension later in life. Progeny of survivors of the Dutch famine in World War II demonstrated a higher prevalence of obesity and diabetes (Ravelli et al., 1976), although this conclusion was questioned by later studies (Jackson et al., 1996; Susser and Stein, 1994). Malnutrition in utero also has been reported to result in increased obesity and its complications later in life (Stanner et al., 1997). Lower birth weights also seem to be associated with increased upper body visceral adiposity in later life with its attendant increased risk of cardiovascular disease (Oken and Gillman, 2003; Rogers, 2003). Since individuals from a lower socioeconomic background are more likely to be exposed to malnutrition during gestation or early childhood, the prevalence of obesity in such subgroups might be expected to be higher.
Adiposity Rebound
Adiposity increases from birth until approximately 1 year of age, then declines to a minimum at approximately 6 years of age. The term adiposity rebound refers to the increase in body mass index (BMI) and body fat that occurs after this nadir in children between the ages of 5 and 7 years. Children experiencing adiposity rebound at an earlier age appear to have a three- to sixfold greater risk of increased adult BMI than do other children (Whitaker et al., 1998). He and Karlberg (2002) demonstrated, through the development of probability charts based on 3,650 children followed from birth to 18 years of age, that children who experienced this rebound before 8 years of age have a higher risk of adulthood obesity. However, Guo and coworkers (2000), using serial BMI data from the Fels Longitudinal study demonstrated that while there was no association between early age at adiposity rebound and adult BMI status in men, after controlling for effects of birth weight, adult physical activity, alcohol and cigarette use, there was approximately twice the risk for overweight with early rebound in women.
Causes of early adiposity rebound have been variously attributed to advanced skeletal maturity (Roland-Cachera et al., 1984; Williams and Dickson, 2002), high protein intake (Roland-Cachera et al., 1995), and parental BMI (Dorosty et al., 2000). Cameron and Demerath (2002) concluded after extensive review of the available scientific literature that the evidence is still not clear about whether age at adiposity rebound is a critical period for the development of obesity, but that early adiposity rebound might well be a statistical predictor of later obesity because of its strong relationship with early adiposity and accelerated maturation, both of which are established markers of later risk of obesity.
Adolescence
Although only 30 percent of adult obesity begins during childhood, 70 percent of the adult obesity that begins in childhood may start during adolescence (Dietz, 1994). Adolescent obesity is associated with a variety of adverse health effects in adulthood, including early mortality in men and increased risks of coronary heart disease, diabetes, and colorectal cancer (Miller, 1988; Must et al., 1992; Wylie-Rosett, 1988). Most of these risks were only slightly attenuated by adjustment for adult obesity, which suggests that obesity during adolescence may determine the risk of these later complications regardless of whether or not the individuals are obese adults.
While total fatness is an important consideration when evaluating developmental aspects of obesity, an additional consideration is adipose tissue distribution. Visceral adipose tissue has an independent effect on obesity-associated comorbidities (Emery et al., 1993) that is separate from that of total body fat, although the developmental aspects of visceral adipose tissue deposition have not been well studied. Among children, visceral adiposity appears to be associated with an increased risk of cardiovascular risk factors such as elevated triglycerides and reduced high-density lipoproteins that are independent of total body fat (Caprio et al., 1996; Gutin et al., 1994). However, the ages at which these relationships appear remain unclear. Cross-sectional studies suggest that visceral adipose tissue deposition is not marked before adolescence, but increases rapidly at that time.
Adulthood
The period after adolescence has not been intensively studied, although approximately two-thirds of adult obesity begins after adolescence. Whether additional critical periods exist in adulthood is less certain, but pregnancy and postpartum may constitute one such period for a subset of women (Williamson et al., 1994). Postpartum weight retention appears to range from 0.5 to 4.8 kg for most women (Johnston, 1991), but African-American mothers may be twice as likely to retain 9.1 kg (20 lb) or more postpartum than Caucasian mothers (Parker and Abrams, 1993). Boardley and colleagues (1995) found that African-American women ate more and were less physically active postpartum than were the Caucasian women in their sample. When the possible confounding factors of prepregnancy weight, gestational weight gain, prenatal physical activity, parity, and socioeconomic status were controlled, African-American women still retained more weight in the postpartum period than did Caucasian women. Results of several recent studies suggest that possible genetic factors may be involved in the tendency to retain weight postpartum. One study found that in women with normal prepregnancy BMIs, high first-trimester serum leptin concentrations (a protein hormone encoded by the obese gene) correlated with increased gestational weight gain and postpartum weight retention (Stein et al., 1998). In another study, women within 12 months of the birth of their first child who were homozygous for the 825T allele of the G-protein 3, considered a thrifty genotype, had significantly higher BMIs and postpartum weight retention than women who did not carry the genotype (Gtersohn et al., 2000). No effect of the genotype was observed among women who had never given birth, suggesting a pregnancy-specific phenomenon. In addition, this relationship was only observed among women who engaged in low levels of physical activity, supporting the idea that physical activity may mitigate effects of genetic endowment on the potential for postpartum weight retention. Whether this particular genetic variation in this specific G protein is causally linked to the observed differences in BMI and weight retention or is merely a marker for the responsible mutation, as well as what the mechanism might be, are both questions that require further investigation (Feldman and Hegele, 2000).
GENETIC DETERMINANTS
The understanding of the genetic influences on overweight and obesity in humans has increased dramatically. Individuals show significant heterogeneity in their body weight and body fatness responses to altered energy balance, dietary components, and changing activity levels. It is now well-established that overweight and obesity have a significant genetic component, with estimates of the contribution of genetic variation to observed variation in obesity-related phenotypes (such as BMI, fat mass, and leptin levels) ranging from 30 to 70 percent (Comuzzie et al., 1993, 1994, 1996). However, little is yet known about the specific causes of heterogeneity (Prusse and Bouchard, 1999). It seems clear that energy metabolism and neural control of appetite are involved in regulating body weight and may contribute to the etiology of obesity. Studies of resting metabolic rate show that the variation within families is less than the variation among families (Bogardus et al., 1986).
Several studies have evaluated the potential mechanisms by which genetic factors may contribute to obesity. One of the mechanisms by which differences in energy metabolism may contribute to obesity may involve defects in uncoupling proteins (UCP). Several types of uncoupling proteins have been identified. Fleury and colleagues (1997) first described human uncoupling protein 2 (UCP-2) and its links to obesity and hyperinsulinemia. Bouchard (1997) noted that markers near the UCP-2 gene in humans are linked to differences in resting metabolic rate. Thus, genetic differences in UCP-2, and perhaps other UCPs, may contribute to human obesity.
There is a group of at least 20 Mendelian syndromes in which obesity is a component, including Prader-Willi, Bardet-Biedl, Borjeson, Cohen, and Wilson-Turner (Gunay-Aygun et al., 1997; Reed et al., 1995). These genetic disorders are rare, and family studies do not suggest that the genes responsible for these syndromes are involved in the common forms of human obesity. For more than 99 percent of obese humans, the genetic basis of their obesity is unknown.
Animal Models of Genetic Obesity
The strongest evidence for genetic weight-regulating mechanisms is the recent elucidation of single gene defects that are associated with excessive weight gain in animals. Single gene mutations can indisputably cause obesity in both rodent models and in humans. In rodents, such mutations have been identified in at least five genes: the obese gene for the circulating adipose tissue-secreted factor leptin; the db gene for the receptor of leptin; the agouti yellow mutation, which controls hair color in mice through the production of melanin pigments (with its human equivalent, agouti signaling protein gene); the fat mutation in the carboxipeptidase E gene, which is a prohormone processing enzyme; and the tub mutation, the function of which has yet to be determined. Of the five gene products that currently have been associated with weight regulation, leptin is the best characterized. Genetic defects in leptin are associated with extreme obesity in both humans and laboratory animals. In addition, serum concentrations of leptin are elevated in close proportion to body fat in obese people with no defect in the leptin gene. Recent studies show that administration of recombinant leptin to lean and obese individuals results in dose-dependent weight loss (Heymsfield et al., 1999). Further research is needed to assess the potential role of leptin in obesity treatment.
Familial Aggregation of Risk for Obesity
Using the comprehensive Danish adoption registry, Stunkard and colleagues (1986) found that adopted children who were raised separately from their biological parents had body weights closer to those of their biological parents than to those of their adoptive parents. The children in this study were separated from their parents at a very early age, generally before 3 months, so the opportunity for the biological parents to instill eating and activity habits was very limited. Another study of adoptees showed a significant genetic influence on obesity, but none of the environmental indicators evaluated were found to contribute, although a number of the conditions considered have previously been associated with obesity (Sorensen et al., 1998). Stunkard and colleagues (1986) estimated that as much as 70 percent of the variance in the occurrence of obesity could be attributed to genetic factors, but other authors have postulated that as little as 20 percent of the variance is due to genetic factors. The general consensus is that genetic factors account for about 30 to 50 percent of the variance in the occurrence of obesity (Bouchard, 1997).
Twin studies provide the most impressive clinical evidence that genetic factors play an important role in the etiology of obesity in humans. Stunkard and colleagues (1990) studied identical and nonidentical twins who were reared together and others who were reared apart. They found a high correlation of body weight among identical twins, even if they were reared apart. Bouchard and colleagues (1990) studied twins who were isolated in the Canadian wilderness with no access to foods other than those provided by the investigators. Identical twins were overfed for a period of 100 days, and their gains in body weight and adipose tissue were evaluated. There was a closer association of both body weight and intra-abdominal adipose tissue (visceral fat) within twin pairs than among twin pairs.
The maximal heritability of obesity has been estimated to range from 30 to 50 percent, based on a review of family studies (Chagnon et al., 2000). Although extensive efforts have been made to identify mutations in the genes identified as obesity-associated in rodents and in other candidate genes for obesity in humans, to date only a handful of individuals have been identified with mutations in any of the genes that have produced obesity in rodents. Specifically, several humans have been identified with mutations in the leptin gene or its receptor, but no individuals have yet been found with mutations in the other genes identified in rodents.
In total, single gene mutations have been identified as responsible for obesity in 25 persons, with these mutations appearing in 7 genes (12 different mutations) (Prusse et al., 1999) or in 5 genes (Chagnon et al., 2000). Studies of quantitative trait loci (QTL) in rodents have suggested at least 98 different QTLs associated with obesity (Chagnon et al., 2000).
Currently, the major effort in the search for specific genes that contribute to human overweight and obesity is based on the use of genome scanning. In genome scanning, linkage analysis is conducted to identify QTLs that affect the specific phenotype under study. The use of genome scanning has provided evidence of QTLs that influence body weight and the number of fat cells (Chagnon et al., 2000).
Comparison of the risks of obesity in spouses and in first-degree relatives has suggested that genetic factors may be of greater prominence in more severe obesity (Katzmarzyk et al., 2000). Among the members of families that contain at least one morbidly obese person, a major gene effect was transmitted in a codominant fashion, suggesting a gene-environment interaction (Rice et al., 1999). Both multifactorial and major gene effects have been suggested. Efforts are ongoing to identify the genetic and molecular basis of overweight and obesity, and it is likely that many genes (and within these genes and their promoters, many different mutations or variants) that are responsible for the genetic variation of obesity in humans will be identified.
The development of obesity likely involves a combination of shared environment and shared genetic propensities. The rapid increase in prevalence of obesity in the United States, as well as in many other countries, across all age groups may reflect a removal of environmental constraints (e.g., high levels of daily activity and food availability) on the expression of obesity genotypes. Knowledge of the genetic components of obesity is not likely to be useful to the military in the near term, but identification of markers of potential risk of obesity may well have implications for future screening.
AGE
Cross-sectional and longitudinal studies indicate a gradual increase in the average BMI of Americans up to the ages of 50 to 60 years (IOM, 1995). This trend is similar, with some variation, across males and females and across all evaluated ethnic groups. Population studies also indicate a decline in body weight and BMI among the elderly, usually in the seventh and eighth decades (IOM, 1995; Kuczmarski et al., 1994; NHLBI, 1998). The same trends have been identified in changes in total body fat and percent body fat (Chumlea et al., 2002). Overweight and obesity thus reach maximal rates among middle-aged adults. This pattern is shown in .
The prevalence (%) of overweight and obesity of men and women by age in the U.S. population. Preobesity = body mass index (BMI) of 2529.9, class I obesity = BMI of 3034.9, class II obesity = BMI of 3539.9, and class III obesity (more...)
The age-related body mass increase up to the fifth and sixth decades is accompanied by additional anatomical, structural, and body compositional changes. Stature declines from about age 30 onward, with rates in women faster than those in men and for postmenopausal women faster than their premenopausal counterparts. Declining stature accounts for a small portion of the age-related increase in BMI (Gallagher et al., 1996).
Many weight-management experts agree that body weight becomes progressively more difficult to maintain with age, but there appears to be little rationale for increasing the upper BMI range consistent with good health as individuals become older. Williams (1997) indicated that body weight and associated circumferences would increase with advancing age unless food intake is reduced and physical activity is substantially increased.
A large number of cross-sectional studies, however, do demonstrate that body fat increases with age, even after controlling for changes in body weight and physical activity levels (Baumgartner et al., 1995; Flynn et al., 1989; Forbes, 1987; Forbes and Reina, 1970; Gallagher et al., 1996, 1997; Noppa et al., 1980; Novak, 1972; Steen et al., 1979). Gallagher and colleagues (1996) demonstrated that the mean body-fat content in nonexercising civilian women with a BMI of 25 increased from 30 percent for those between the ages of 17 and 20 years to 36 percent for those ages 40 years and older. The implication of this is that lean body mass and, frequently, skeletal mass, decrease with age. Additionally, partitioning of adipose tissue between the subcutaneous and visceral compartments is also moderated by age (Borkan et al., 1983). Men have more visceral adipose tissue than do women at all ages, and the rate of visceral adipose tissue increase with age is greater in men than in women (Blaak, 2001).
In contrast to body fat, skeletal muscle mass declines with age beginning around the third decade of life (Dutta and Hadley, 1995). This observation is true not only for the general population, but it is also evident in military personnel (USAF, 1975). The rates of decline may accelerate after the onset of menopause in women (Aloia et al., 1991) and for both genders in the seventh and eighth decades (Flynn et al., 1989). Losses of skeletal muscle parallel changes in skeletal minerals with advancing age and are present even after controlling for loss in body weight (Gallagher et al., 2000). The mechanisms of body composition change with aging are multifactorial and include physical inactivity, diet, and hormonal and cytokine alterations. The loss of lean mass and gain in fat mass occur even with no apparent change in body weight. Since lean mass contributes the larger share of metabolic activity, total energy expenditure during rest or low activity will also decrease proportionally with the loss of lean mass.
Total energy expenditure and thus, energy requirements, decrease with advancing age (Tzankoff and Norris, 1978). Physical activity levels are lower in older individuals, which account for a portion of the energy expenditure reduction that comes with aging. Resting energy requirements are also lower in the elderly, due largely to decreases in all metabolically active tissues, including skeletal muscle, brain, and visceral organs. In laboratory animals, the heat produced by tissues per unit of mass decreases with age (a decrease in the specific resting energy expenditure of organs), but it remains uncertain whether this observation also applies to humans. The practice of resistance training by people over the age of 50 years may enhance fat-free mass, primarily skeletal muscle, and thereby help offset the age-related decline in resting metabolic rate (Hill and Saris, 1998; Tzankoff and Norris, 1977). In women, loss of ovarian function accounts for a lower rate of overall heat production compared with that observed in premenopausal women (Poehlman and Tchernof, 1998). Thus, both older men and women have lower rates of energy expenditure and, unless counterbalanced by increased physical activity and reduced food intake, older individuals, in general, will gain weight over time.
RACE/ETHNICITY
Whether there are racial/ethnic differences in response to the various components of weight management is a legitimate research question that has been explored to only a moderate extent. Data from National Health and Nutrition Examination Surveys (NHANES) clearly indicate that there are racial/ethnic differences in the prevalence of overweight and obesity. Flegal and coworkers (2002), reporting on 19992000 NHANES data, determined that in men 20 years of age and older, the prevalence of overweight (BMI 25) was 67.4 percent for non-Hispanic whites, 60.7 percent for non-Hispanic blacks, and 74.7 percent for Mexican Americans. The differences were not statistically significant, but sample sizes were relatively small. However, for women ages 20 years and older, the prevalence of overweight was 57.3 percent in non-Hispanic whites, 77.3 percent in non-Hispanic blacks, and 71.9 percent in Mexican Americans. The difference in prevalence between non-Hispanic white and non-Hispanic black women was statistically significant (Flegal et al., 2002). The causes of these differences in the prevalence of overweight have not been clearly identified, but are likely to be a combination of physiology, culture, and behavior.
The relationship of BMI to percent body fat is also affected by race/ ethnicity. Fernandez and colleagues (2003) recently reported the results of an analysis of 11 cross-sectional studies involving body composition assessments of African-American men and women, Hispanic-American men and women, and European-American men and women. The average age ranged from 42.6 to 50.8 years, and the average BMI ranged from 25.1 (European-American women) to 29.8 (African-American women). Total body fat was measured using dual-energy X-ray absorptiometry. There were no differences in the estimation of percent body fat from BMI for men across ethnic groups. However, for women with BMIs less than 30, Hispanic-American women had a significantly higher percent of body fat at a given BMI than did African-American or European-American women. However, at BMIs greater than 35, European-American women had a higher percent body fat than either of the other two groups of women. Some earlier studies have reported greater fat free mass in African-American women compared with Caucasian women with the same BMI, primarily due to the greater skeletal mass in African-American women (Gallagher et al., 1996; Ortiz et al., 1992).
A number of studies have examined possible physiological reasons for these race/ethnic differences. Foster and colleagues (1997) explored differences in resting energy expenditure (REE) between obese African-American women and Caucasian-American women. They found that REE was most closely correlated to body weight and that African-American women had lower REE than Caucasian-American women. Melby and coworkers (2000) examined behavioral and physiological characteristics related to obesity risk in young, sedentary, nonobese African-American and Caucasian-American women. The two groups were similar in age and anthropometric characteristics. Parameters examined included REE, respiratory exchange rates (RER), insulin sensitivity, and maximal oxygen consumption. REE was 3 to 4 percent lower in African-American women, but the difference was not statistically significant. However, the resting RER was significantly lower in African-American women. The African-American women also had significantly lower insulin sensitivity values that resulted in higher acute phase insulin response to glucose. Total daily energy expenditure and physical activity energy expenditure were significantly lower in the African-American women.
Tanner and coworkers (2002) recently identified a relationship between muscle fiber type and obesity. In a study of lean and obese African-American and Caucasian women, type I muscle fibers (slow twitch, oxidative muscle fibers) were significantly reduced in obese women compared with the lean women, and type IIb fibers (fast twitch, glycolytic muscle fibers) were significantly increased. These differences between lean and obese women were greater in African-Americans than in Caucasians. The type IIb phenotype is insulin resistant and deficient with regard to lipid disposal. The authors speculated that the prevalence of the type II fibers might result in partitioning lipid toward storage in skeletal muscle or adipose tissue rather than oxidation within the skeletal muscle, resulting in a positive fat balance.
A number of studies have also examined social and behavioral factors that may contribute to the difference in the prevalence of overweight between African-American and Caucasian women (Kumanyika et al., 1993; Stevens et al., 1994). Attitudinal and behavioral factors that limit the ability of some African-American women to lose weight or maintain weight loss have been identified. Regardless of whether or not they were overweight, African-American women were half as likely as Caucasian women to consider themselves overweight. There is a much greater cultural tolerance of overweight among African-Americans, and they have different body image perceptions. Although African-American women responded physiologically to a weight-reduction program in the same manner as Caucasian women, their drop-out rate from the program was double that of Caucasian women (Glass et al., 2002).
PHYSICAL ACTIVITY
While recent studies point to the importance of genetic factors in the etiology of obesity (Bouchard, 1997; Chagnon et al., 2000), the rapid rise in the prevalence of overweight and obesity in the last 20 years likely reflects major environmental shifts in exercise habits and food availability, which can be controlled.
Physical activity represents an important component of volitional energy expenditure. Modern transportation and other conveniences have reduced the need for energy expenditure in the form of physical exertion. Reductions in physical activity over the past several decades likely contribute to the evolution of the positive energy balance and weight-gain characteristics of all industrialized societies. Lack of physical activity begins in youth, with television watching time correlated with BMI, as well as with both prevalence and severity of overweight (Dietz and Gortmaker, 1985; Katzmarzyk et al., 1998; Tanasescu et al., 2000). A reduced emphasis on school physical education classes has been accompanied by a gradual decline in childhood fitness (Luepker, 1999). Indeed, physical inactivity is a major risk factor for development of obesity in children and adults (Astrup, 1999; Goran, 2001). Among adults who have maintained weight loss over time, a common factor is increased physical activity (Klem et al., 1997).
The effects of physical activity on weight and health may be influenced by age. Owens and coworkers (1992) evaluated the effects of physical activity on both weight change and the risk factors for cardiovascular disease during the perimenopausal period. Women who increased their activity levels during the 3-year study period (as measured using the Paffenbarger Physical Activity Questionnaire) had the smallest increases in body weight and the smallest decrement in high-density lipoprotein cholesterol.
Flatt (1987) has pointed out that to avoid increased fat deposition, both energy balance and macronutrient balance (especially fat balance) are necessary. When dietary fat is elevated, there is limited capacity to reduce total body fat by fat oxidation. Exercise, especially in bouts of 30 minutes of activity or more (Pate et al., 1995), can promote fat oxidation because the substrate that is preferentially oxidized switches from carbohydrate to fat. Thus, chronic extended bouts of exercise may, in effect, substitute for expansion of the adipose tissue, allowing the physically active individual to achieve fat balance while maintaining a lower body-fat mass than the sedentary individual (Flatt, 1987). Jakicic and coworkers (1995) initially demonstrated that over the short term, four 10-minute bouts of exercise per day, four times per week is more effective in reducing body weight than a single 30 to 40 minute period of exercise. However, the long-term data indicated that the short-term bouts of exercise were not as effective as the long bouts in reducing weight and maintaining weight loss (Jakicic et al., 1999).
Most fatty-acid oxidation in the human body occurs in muscle (Calles-Escandon and Poehlman, 1997). The intrinsic capacity of muscle to oxidize fat can be impaired by physical inactivity and possibly by loss of estrogen in women, but it is amenable to partial correction by exercise training (Calles-Escandon and Poehlman, 1997). A decrease in aerobic capacity and fat-free mass, rather than aging per se, is responsible for the decrease in fat oxidation seen in elderly women (Calles-Escandon and Poehlman, 1997). Exercise training increases oxidative disposal of fatty acids and improves muscle metabolism in both young and old individuals. However, the elderly do not increase fat utilization in response to exercise to the same extent as the young, despite performing exercise to the same intensity and for the same duration (Blaak, 2000; Calles-Escandon and Poehlman, 1997).
In a study of 970 healthy, female twins with a wide range of percent body fat, both total body fat and central adiposity were associated with physical activity (Samaras et al., 1999). Moderate-intensity sports of 1 and 2 hour durations accounted for within-pair differences of 1.0 kg and 1.4 kg, respectively, of total body fat. Among participants in whom one of a pair of twins was overweight, higher levels of physical activity were still associated with 3.96 kg lower total body fat and 0.53 kg lower central abdominal fat. In other words, even persons with an apparent genetic predisposition to adiposity showed an effect of physical activity on body-fat mass (Samaras et al., 1999). Studies of energy expenditure in individuals and families show that differences are greater between families than within families (Bogardus et al., 1986). Some differences in energy expenditure between families are due to genetic factors and some are due to differences in activity patterns.
Hormones affect the relationship of physical activity, body fat, and fat-free mass. Guo and coworkers (1999) found that associations between physical activity and fat-free mass were more pronounced in postmenopausal women than in premenopausal women, and that hormone replacement therapy had beneficial effects on body composition. Monozygotic twin pairs who were concordant for smoking and hormone replacement therapy status, but discordant for moderate-intensity activity, showed greater within-pair differences in total body fat than those who were concordant for activity level (Samaras et al., 1999), suggesting that the effect of physical activity is greater than that of hormonal status.
Habitual physical activity also affects other physical characteristics. Gilliat-Wimberly and coworkers (2001) found that an association exists between habitual physical activity and maintenance of resting metabolic rate in middle-aged women. Physical activity also may reduce the incidence of chronic diseases by favorably altering blood lipid profiles, reducing body fat, and improving lean body mass (Eliakim et al., 1997; Schwartz et al., 1991; Wei et al., 1997; Wilbur et al., 1999).
FOOD
Intake
In conjunction with the importance of physical activity levels, energy intake must be matched to energy expenditure. Positive energy balance results if energy intake is greater than energy expenditure. Increased energy consumption, decreased energy expenditure, or both can result in positive energy balance.
While the etiology of obesity is multifactoral, the common characteristic of all obese people is excessive energy storage in the form of body fat. Whether obese people consume more energy than do lean people has been a major source of controversy. Studies in modern respiratory chambers using doubly-labeled water have shown that weight-stable obese people have a higher resting metabolic rate and total 24-hour energy expenditure than do lean people (Jequier and Schutz, 1983; Ravussin et al., 1982; Zed and James, 1986), which demonstrates that average energy intake must indeed be higher in the obese. Some differences in energy expenditure, and consequently in energy intake, among families are due to genetic factors and differences in activity patterns. Social and cultural factors also contribute to individual food intake differences (de Castro, 1999).
Since the energy in food is derived from the macronutrients protein, fat, and carbohydrate (CHO), plus the optional energy source, alcohol, diets that are high in fat tend to be low in complex CHOs such as fiber. There is still considerable controversy over whether the role of diet composition or simply total energy intake is important in maintaining a healthy body weight.
Composition
A high energy intake or an energy intake that is not adjusted downward with declining physical activity or age-related decreases in lean body mass is associated with the development of overweight or obesity in susceptible individuals. In addition to total energy intake, the character of the diet may play a role in the etiology of obesity. High-fat diets may promote increased energy intake or may be associated with metabolic changes that promote the deposition of adipose tissue.
Dietary Fat
Research in both animals and humans suggests that high-fat (low in complex CHOs) diets promote obesity (Astrup et al., 2000; Bahceci et al., 1999; Blundell and Cooling, 2000; Cheverud et al., 1999; Maffeis et al., 2001). Because fat is more energy dense than other foods (9 kcal/g versus 4 kcal/g for protein and CHO), eating high-fat foods results in a greater energy intake than would eating a similar quantity of lower-fat foods. Fat modifies the taste of food and, in some people, promotes excess intake. Fatty foods tend to be easier to chew or may not require chewing, thus making larger quantities easier to eat in a shorter time than foods that require more mastication. Dietary fat also has a weaker satiation effect than CHOs, which results in the over consumption of fat (Rolls and Hammer, 1995; Rolls et al., 1999).
Some of the difference in weight gain on a high-fat versus a low-fat diet may be explained by differences in the metabolic processing of fat. Compared with dietary fat, CHOs require additional energy expenditure for digestion, assimilation, and conversion to fat. When energy intake exceeds expenditure, 23 percent of energy consumed is required to convert and store CHO as fat, compared with only 3 percent to store fat. Two studies in laboratory animals have demonstrated this effect of dietary fat on body weight and body composition (Donato and Hegsted, 1985; Lin et al., 1979).
The link between dietary fat and obesity in humans is not conclusive because of difficulties in accurately measuring or controlling the food intake and energy expenditure of individuals and the need to rely on estimates of body composition. Nonetheless, increasing evidence from clinical studies suggests that dietary fat promotes weight gain in humans as well as in animals. Studies in which people were overfed diets varying in the proportion of energy from fat (40 to 53 percent of kcal as fat) showed that high-fat diets promoted weight gain more efficiently than did lower-fat diets (Sims et al., 1973).
A positive correlation between the proportion of fat in the diet and the incidence of obesity has been noted among various cultures, as well as within ethnic groups that have migrated to the United States and adopted American dietary patterns (Curb and Marcus, 1991; Kushi et al., 1985). While these correlations all point to a causal role for dietary fat in obesity, they are subject to confounding variables such as differences in energy intake and expenditure, health status, and genetic and environmental influences. However, based on information such as that described above, Danforth (1985) recommended shifting to a higher-CHO and lower-fat diet to reduce the high prevalence of obesity in affluent societies such as the United States.
Obesity is more closely correlated with the level of dietary fat than with total energy intake (Dreon et al., 1988; Romieu et al., 1988). A low incidence of obesity has been observed among vegetarians who typically consume low-fat, high-CHO diets (Knuiman and West, 1982; Sacks et al., 1975). However, those who adhere to vegetarian diets for religious rather than nutritional reasons probably have a higher-fat diet (Dhurandhar and Kulkarni, 1993), and the prevalence of obesity among these types of vegetarians is high compared with that of omnivores (Dhurandhar and Kulkarni, 1992).
Some studies have failed to demonstrate an association between fat intake and body weight in free-living populations. On the basis of food frequency questionnaires, Macdiarmid and colleagues (1994) stratified 1,800 people by their fat consumption (high was considered to be 45 percent or more kcal as fat and low was considered to be 35 percent or less kcal as fat) and found no statistically significant difference in age, BMI, or social class between the two groups. However, the high-fat group rated their general diet and health as poorer. The high-fat group also consumed significantly more protein and total energy, but less CHO and fiber; consumed meat and high-fat dairy products more frequently; and consumed fewer fruits, vegetables, and cereals.
Results of a small study suggest that the amount of energy required to maintain body weight may be related to the proportion of fat in the diet, regardless of an individual's weight status (Prewitt et al., 1991). These findings suggest that dietary fat may promote greater weight gain and body-fat accumulation than expected on the basis of energy intake alone. In contrast, Leibel and colleagues (1992) found no relationship between the ratio of dietary fat/CHO and the total energy required to maintain body weight. CHO ranged from 15 to 85 percent of total intake, and kcal from fat ranged from 0 to 70 percent of total intake. The disparity between findings of these two studies may be due to the shorter duration of the second study (33 days average and ranging from 15 to 56 days compared with 140 days in the Prewitt study). Differences among the normal-weight patients in the study of Prewitt and colleagues (1991) were not seen consistently before 13 to 16 weeks. Also, body composition was not assessed in the Leibel study, and results of animal studies suggest that isocaloric diets of varying fat content may produce differences in percent of body fat without changing body weight (Boozer et al., 1990, 1993).
The arguments for whether dietary fat promotes obesity were summarized in two recent, competing editorials. Willett (1998a, 1998b) argues that obesity has increased in the United States despite reductions in intake of dietary fat and that ecological studies have found no relationship between fat intake and obesity. In contrast, Bray and Popkin (1998) argue that individuals who gained weight may not have decreased (or may have increased) their intake of dietary fat. They also argue that ecological studies may not be appropriate to study the relationship between fat intake and obesity, that body weight is a poor measure of body fatness, and that most of the previous studies focused on outcomes other than obesity. Although the literature is not clear, results of studies on laboratory animals and the small number of human studies suggest that dietary fat does promote obesity. Recently, Astrup and colleagues (2002) reviewed evidence on the effects of low-fat diets. Four meta-analyses of weight change occurring on low-fat diets in intervention trials with overweight subjects were reviewed. These analyses consistently demonstrated significant weight loss in both normal-weight and overweight subjects.
Carbohydrates
Several rationales have been postulated for the use of high-protein, low-CHO diets: (1) intake of a high proportion of kcal as CHO has adverse physiological consequences, such as increasing insulin secretion, promoting fat deposition, and increasing serum triglycerides levels; (2) low-CHO diets can lead to a ketogenic state, which has been hypothesized to suppress appetite; (3) a high-protein diet preserves lean body mass during weight loss; and (4) the thermogenic effect of protein is the highest of the three macronutrients, resulting in increased energy expenditure for a similar intake.
There is at least some scientific rationale for the above hypotheses (Skov et al., 1999a, 1999b). A high-protein diet has been found to: stabilize blood glucose during nonabsorptive periods and reduce insulin response following test meals (Layman et al., 2003b), improve glucose oxidation (Piatti et al., 1994), decrease lipid oxidation (Piatti et al., 1994), produce positive changes in blood lipids (Layman et al., 2003b), and provide greater satiety than diets higher in CHO (Layman et al., 2003a). Although more research is needed on the subject of amino acid flux measurements and how it relates to blood glucose levels, data from Layman and colleagues (2003b) support the idea that the ratio of dietary protein and CHO can have a significant effect on metabolic balance and specifically on glucose homeostasis during weight loss.
The role of CHO in soft drinks in producing obesity is controversial. Some studies suggest that an increase in the consumption of soft drinks may have contributed to the increased prevalence of obesity (French et al., 2000; Troiano et al., 2000), whereas others do not support this hypothesis (Gibson, 2000; Macdiarmid et al., 1998; O'Brien et al., 1982).
Portion Size
There is little research available on the role of portion size in the increasing prevalence of overweight in the United States. However, common sense dictates that it is a contributing factor. For example, a single serving of meat is considered to be 3 to 4 oz based on the Dietary Guidelines and the U.S. Food Guide Pyramid. However, in restaurants (where Americans are spending a greater portion of their food dollars), an 8-oz portion of red meat would be considered a petite serving; the standard serving would be 12 to 16 oz. Thus, an individual consuming a 16-oz steak in a restaurant would be likely to report (if asked in a dietary survey) consuming a single serving of red meat, when in reality 4 to 5 servings were consumed.
The intake of soft drinks has increased dramatically in the last 40 years, as has the trend towards larger portion sizes (Hill and Peters, 1998). While a standard serving of a soft drink in 1960 consisted of one 6-oz serving, the standard size serving today is 12 oz, and many vendors sell 20-oz bottles almost exclusively. Fountain drinks have also increased to the super-jumbo 32- to 64-oz sizes. It is not unusual for individuals to consume some 500 to 1,000 kcal per day from soft drinks in addition to their usual solid-food diet.
The change to larger portion sizes has been particularly apparent in fast-food restaurants where portion size has been used as a competitive tool. Full-service restaurants also have adopted the practice of serving larger meals. Similar to the increase in soft drink portions sizes, fast-food restaurants now offer super-size portions for a minimal increase in cost. For example, a jumbo super-size order of a large hamburger, french fries, and soft drink at a fast-food restaurant may now contain more than 1,500 kcal for a single meal (Nielsen and Popkin, 2003; Young and Nestle, 2002, 2003). One of the distinguishing features of dining out in Europe compared with the United States is the difference in restaurant portion sizes, a factor that may contribute to the lower prevalence of obesity in Europe.
A recent trend analysis of portion size was conducted by Nielsen and Popkin (2003). Data were taken from four national food-consumption surveys covering the period 1977 to 1996. Food consumption was estimated as energy intake in kcal and as average portion sizes using food models to assist respondents in identifying portion size. Results demonstrated that for foods eaten both inside and outside the home, portions sizes have increased for salty snacks, desserts, soft drinks, fruit drinks, french fries, hamburgers, cheese-burgers, and Mexican food.
Meal Patterns and Eating Habits
Eating patterns that are appropriate for an active lifestyle may continue after the individual changes to a more sedentary lifestyle. Individuals for whom this observation has been made include athletes and a large percentage of people with increasing age and changing occupational responsibilities. Athletes who are in training expend large amounts of energy each day and, for many organized sports, are encouraged to eat large quantities to maintain their weight at an artificially high level. When activity declines, the eating pattern established during training may not be adjusted to meet the new lower energy needs. The same is true of military personnel. During initial entry training, advanced individual training, and special forces training, large amounts of energy are expended on a daily basis. By the time training is completed, individuals have been habituated to consume large amounts of food over a very short period of time.
In many occupations, tasks that require more physical activity are assigned to younger workers. As these workers age and acquire more responsibility, their work may become more sedentary, but eating patterns may not change. This pattern of decreased occupational energy expenditure with job promotion may be common in the military as well. Privates, airmen, and junior noncommissioned officers are more active than senior officers and noncommissioned officers. Despite strong commitments to engage in daily physical fitness, which may be unchanged or even increased in more senior individuals, the decrease in activities of daily living and job performance can lead to a positive energy balance unless particular care is taken to reduce energy intake.
The ubiquitousness of vending machines and fast-food outlets ensures constant access to foods at workusually foods with a high caloric content largely in the form of fat or refined CHO. A major contributing factor to the epidemic of obesity in recent years is likely the rise in the proportion of meals eaten away from home (eating out), along with the increase in access to foods in virtually all locations. These changes have contributed in several ways to promoting obesity. Because more families include two-wage earners, adults spend more time out of the home and do not have time to prepare meals as they customarily did in the past. Meals consumed at restaurants tend to be larger and have a higher caloric content than those consumed at home, mainly because of higher fat content and larger portion sizes (Young and Nestle, 2003). In addition, a high percentage of meals eaten away from home are eaten in fast-food restaurants or consist of fast-food take-out. The presence of food in virtually every circumstance of daily life, from fast-food outlets to vending machines, encourages and allows individuals to consume multiple calorically dense meals and snacks per day (Bell et al., 1998; Rolls, 2000).
PHYSIOLOGICAL FACTORS
A number of phenotypic characteristics have been associated with the risk of weight gain, notably alterations in nonvolitional components of energy expenditure. Energy expenditure can be divided into three main components:
Resting metabolic rate (RMR), the rate of energy expended at rest, under thermo-neutral conditions, and in a post-absorptive state.
Thermic effect of feeding, the incremental increase in energy expenditure after a meal is consumed due to the energy costs of absorption and the transport of nutrients, as well as the synthesis and storage of protein, fat, and CHO. Some of the thermic effect of feeding may be mediated by sympathetic nervous system activity.
Energy expended for physical activity, including involuntary movements associated with shivering, fidgeting, and postural control.
RMR accounts for 60 to 75 percent of total energy expended in most adults. RMR is primarily related to the maintenance of fat-free mass, reflecting such activities as protein synthesis and breakdown, temperature and cellular homeostasis, and cardiovascular, pulmonary, and central nervous system function. Metabolism associated with visceral organ mass makes the largest contribution to RMR, followed by that of skeletal muscle mass and adipose tissue (Gallagher et al., 1998). RMR is consistently greater in men than in women due to the greater lean tissue mass of males. A low RMR relative to body size was found to predict weight gain (Ravussin et al., 1988) in both men and women, although some studies have not confirmed this observation (Weinsier et al., 2000). RMR begins to decrease with age in the middle of the fourth decade. Gilliat-Wimberly and coworkers (2001) found that an association exists between physical activity and maintaining RMR in middle-aged women.
The thermic effect of feeding usually accounts for 5 to 10 percent of daily energy expenditure and varies between lean and obese individuals (Astrup, 1996). Extensive studies have been inconsistent in supporting the view that excessive weight gain is secondary to a reduced thermic effect of food (Tataranni et al., 1995).
Recent studies support the view that small, nonvolitional physical activities such as fidgeting may account for individual differences in energy expended with changes in energy balance (Levine et al., 1999; Zurlo et al., 1992). Although relatively small in caloric magnitude, these activities may account for some of the between-individual differences observed in the regulation of body weight.
These three phenotypic energy expenditure characteristics serve as markers for potential weight gain over the long term. Many factors may contribute to these individual energetic differences, and the origin of these differences is the basis of intensive study.
ENVIRONMENTAL FACTORS
Smoking and Alcohol
Cigarette smoking increases metabolic rate and may limit food intake, and weight gain is a common consequence of smoking cessation (Perkins, 1993; Russ et al., 2001). The use of alcoholic beverages may also have an impact on body weight. Energy consumed as alcohol that is in excess of need is converted to and stored as fat. Drinking alcohol has been shown to be associated with a greater energy intake than drinking nonalcoholic beverages, perhaps due to increased appetite (Tremblay and St-Pierre, 1996; Tremblay et al., 1995).
A recent, large prospective study of a cohort of men ages 40 to 59 with a 5-year follow-up found that mean BMI increased significantly from the light-to-moderate to the very-heavy alcohol intake group. The study concluded that heavy alcohol intake (defined as 30 g/day of alcohol) contributed directly to weight gain and obesity, regardless of the type of alcohol consumed (Wannamethee and Shaper, 2003).
Pharmacological Agents That Produce Weight Gain
Numerous drugs can produce weight gain and fat gain. These include glucocorticoids (e.g., prednisone), hypoglycemic agents (e.g., insulin, sulfonylureas), certain antihypertensive agents (e.g., prazocin), anti-allergens (e.g., cyproheptadine), and numerous drugs that affect the central nervous system (e.g., thorazine, tricyclic antidepressants, valproic acid, lithium). Most of these drugs are used for diseases that mandate separation from the military, but there are a number of drugs that may be taken by military personnel that are not deemed a rationale for separation.
SOCIAL FACTORS
Americans live in a culture in which food is abundant. A well-developed and efficient food transportation and storage system assures a readily available and affordable food supply throughout the entire year.
The relative affluence of Americans has led to an increase in consumption of snack foods (Morgan and Goungetas, 1986) and an increase in the proportion of foods of animal origin compared with that of foods of plant origin (Senauer, 1986). Foods of animal origin are likely to be higher in energy and fat than comparable quantities of foods of plant origin.
The availability and abundance of food in the U.S. marketplace has accelerated dramatically in the past 30 years. The per capita energy content of food entering the American marketplace increased about 500 calories on a daily basis during this time period. In addition, fat intake has also increased steadily, although the relative intake of fat has been decreasing since the 1970s (Putnam and Allshouse, 1999). This decrease in fat intake has been associated with an increase in average total energy intake (Bray and Popkin, 1998). Food-supply studies indicate that the increase in the number of calories consumed is accompanied by a shift in macronutrient consumption that reflects an increase in refined CHO consumption and a decrease in consumption of fruits and vegetables (Putnam and Allshouse, 1999).
Family and Ethnicity
Eating is an intensely social activity, and many eating habits are acquired in a familial or ethnic setting. People tend to imitate the eating habits of their parents, so quantity and quality of foods eaten and meal patterns tends to be established early. Traditions that arise around eating patterns in a more agrarian or active society may favor excess consumption. Ethnic groups differ in their perceptions about appropriate body size and what constitutes overweight (Bhadrinath, 1990; Root, 1990).
Studies of changes in diet with immigration and acculturation show, for example, that Japanese who migrated to California and Hawaii have tended to abandon the traditional low-fat Japanese diet for American food patterns (Burchfiel et al., 1995; Curb and Marcus, 1991; Goodman et al., 1992; Hara et al., 1996; Ziegler et al., 1996). The result has been a marked increase in weight among these immigrants. Similarly, Japanese children who remain in Japan, but whose diet is increasingly western, are also getting heavier (Murata, 2000; Takada et al., 1998). Thus, dietary change is strongly associated with increased weight in both of these carefully studied population groups. The same phenomenon is observed in studies of South Asians who have migrated to the United Kingdom and who have modified their diet and physical activity patterns (McKeigue et al., 1992).
Socioeconomic Status
Social class and socioeconomic status (SES) influence the prevalence of overweight. In many countries of the world, lower SES is linked to increased body weight (Molarius et al., 2000). In contrast, in some developing countries and primitive societies, obesity is considered a sign of affluence or fertility (Molarius et al., 2000). However, some researchers who contend that obesity decreases economic status have disputed the belief that lower SES causes obesity in the United States. For example, one study reported that women who were overweight in late adolescence or early adult life were more likely to have lower income, greater levels of poverty, and decreased rates of marriage than were normal-weight women with comparable degrees of disability (Gortmaker et al., 1993).
The Potential Role of Viruses in the Etiology of Obesity
The possibility exists that at least some cases of human obesity are due to viral infection. Five viruses and scrapie agents cause obesity in animals (Bernard et al., 1988, 1993; Carp et al., 1998; Carter et al., 1983a, 1983b; Dhurandhar et al., 1990, 1992, 1997, 2000; Gosztonyi and Ludwig, 1995; Lyons et al., 1982; Nagashima et al., 1992). One of these viruses is a human adenovirus, Ad-36, which has been shown to produce a syndrome of increased body fat and paradoxically decreased serum cholesterol and triglycerides in chickens and mice (Dhurandhar et al., 2000). Preliminary data have been reported that demonstrated similar results in monkeys (Atkinson et al., 2000). Other preliminary studies suggest that humans with serum antibodies to Ad-36 have a higher BMI and lower serum lipids than do Ad-36 antibody-negative individuals (Atkinson et al., 1998).
Humans in Bombay, India, who had serum antibodies to SMAM-1, an avian adenovirus, were noted to be significantly heavier and to have lower serum lipids compared with antibody-negative individuals. Viral antigen was found in the serum of two of the individuals with SMAM-1 antibodies (Dhurandhar et al., 1997).
More research is needed to confirm the hypothesis generated from the above data that some cases of human obesity might be due to a viral infection. Since adenoviruses are common cold viruses, the possibility of the spread of Ad-36 and perhaps other obesity-producing viruses in the military community may be of significant concern.
SUMMARY
The brief review of factors influencing body weight presented in this chapter demonstrate that maintaining a healthy body weight is an extremely complex issue. Maintenance of fitness and appropriate body-fat standards by military personnel is affected by each individual's genetics, developmental history, physiology, age, physical activity level, environment, diet, ethnicity, and social background.